John A. Medeiros
![]()
What has been proven is that there are multiple cone pigments. In fact, more than three. However, the existence of multiple pigments is a separate issue from multiple cone types. In the currently accepted model of color vision there must be exactly three cone classes, each containing its own unique photopigment. The fact is, this has not been proven. The literature generally focuses on three of these pigments as if they are uniquely sequestered in the three cone types. These three pigments are maximally absorbing at around 560, 530, and 450 nm and are usually referred to as the L (long), M (middle), and S (short) wavelength pigments. They are so-named since what should be the red pigment is in fact in the greenish-yellow part of the spectrum. Moreover, their maximum sensitivities are rather poorly distributed for three-dimensional color vision spanning the spectrum to which the eye is sensitive.
While it has been relatively easy to extract and characterize the rhodopsin photopigment of the rods, it has proven to be very difficult (i.e., impossible) to directly find the cone pigments in the primate retina by actual extraction. Their best identification has been through the use of a number of indirect techniques including molecular genetics, microspectrophotometry, and reflection densitometry. We examine here what has been determined by each of these methods.
Perhaps the best approach to identify the cone photopigments is by identification of the DNA machinery that codes for the protein opsin portion of the pigments (Nathans, et al, 1986). This has worked well, but perhaps too well since this line of research has found an entire array of pigments with absorption spectra that have peaks spanning the red-green range (c.f. Neitz & Neitz, 1995). In any event, the results from the molecular genetics work only shows that the machinery exists to make multiple pigments. It does not prove there are multiple cone types. Multiple pigments could, after all, be used to augment some entirely different way to provide color discrimination in the human eye
So, if molecular genetics can’t exactly tell us about cone classes, what about the results from microspectrophotometry (MSP) on the retina (Marks, et al, 1964; Brown & Wald, 1964; Bowmaker, et al, 1980)? In MSP, one shines a small spot of light on an excised (dead) piece of retinal tissue, ideally a single cone, and measures the light transmitted as a function of the illuminating wavelength. The retinal tissue is then bleached by a high-intensity light and the spectral scan is then repeated on the bleached receptors. The difference in transmitted light before and after the bleach is interpreted as the absorption spectrum of the photopigment in the cone.
There were suggestions in the early MSP measurements that both red and green absorbing pigments are present within the same cone (c.f., Marks, Dobelle, and MacNichol, 1964). Many other lines of evidence also suggest this pigment co-expression (Jacobs, et al, 2004; Lukáts, et al, 2002; Lukáts, et al, 2005; Parry & Bowmaker, 2002; Röhlich, et al, 1994; Williams, et al; 2005). If there is more than one pigment within the cone, the light transmitted depends critically on illumination conditions, pigment densities and distributions. It also depends importantly on the waveguide characteristics of the cones and how light is coupled into the cone structure.
It is well known that these MSP measurement are notoriously difficult to do. In the earliest attempts to measure cone photopigments through MSP where success was reported (Marks, et al, 1964; Brown & Wald, 1964) the probe beam was directed axially through the photoreceptors in order to ma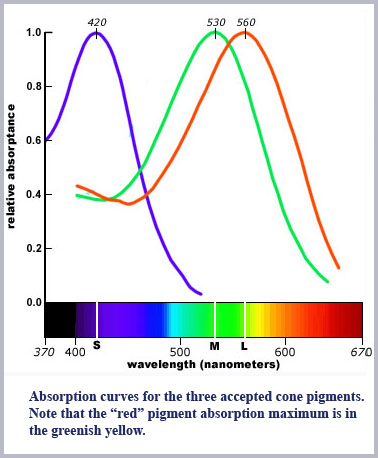 ximize the path length through the pigment. These measurement efforts suffered some severe problems. One critical difficulty was the high levels of light scattering through the retinal tissue as a result of postmortem changes. An additional problem was the mismatch between the numerical aperture of the microscope optics employed and the acceptance angle of the cone waveguide structures they were probing. Liebman (1972) reviewed these measurements and commented: “Unfortunately, almost none of the original data has ever been shown in reports on primate pigments, and no mention has been made of the unacceptable experimental conditions that have been tolerated.” Further, in regard to the peak spectral sensitivities and optical densities reported he concluded: “... the MSP data alone can not be regarded as accurate to better than 20 to 30 nm, and published densities can not be regarded as indicative in the least of what exists in the living eye.”
ximize the path length through the pigment. These measurement efforts suffered some severe problems. One critical difficulty was the high levels of light scattering through the retinal tissue as a result of postmortem changes. An additional problem was the mismatch between the numerical aperture of the microscope optics employed and the acceptance angle of the cone waveguide structures they were probing. Liebman (1972) reviewed these measurements and commented: “Unfortunately, almost none of the original data has ever been shown in reports on primate pigments, and no mention has been made of the unacceptable experimental conditions that have been tolerated.” Further, in regard to the peak spectral sensitivities and optical densities reported he concluded: “... the MSP data alone can not be regarded as accurate to better than 20 to 30 nm, and published densities can not be regarded as indicative in the least of what exists in the living eye.”
In order to circumvent the limitations inherent in probing the cones longitudinally, MSP measurements have also been conducted by transverse probe beams on cones teased from excised retina (Bowmaker, et al, 1978: Bowmaker, et al, 1980). While this technique avoids some of the difficulties inherent in longitudinal probing, it does suffer the problems of both very short path length through the cones and that only one measurement per cone is possible. That is, the technique must necessarily measure the transmission of a light beam before and after a strong bleaching light clears out all the photopigment in a (dead) cone. There is no chance to get repeat measurements and determine if there are perhaps more pigments present in any one cone. If there is more than one photopigment in a cone, there will then be no opportunity to determine how they might be distributed.
Despite these difficulties, there is a general concurrence in the field that the results from MSP measurements and the molecular pigment genetics point to the existence of the three cone photopigments as displayed in the plot above. Recall, however, there remains substantial uncertainty about exclusive occupancy in a given cone with a given photopigment. In addition, evidence from both MSP and pigment genetics point to the existence of a range of photopigments in the 520 to 565 nm range (not just the two usually plotted).
If both pigment genetics and MSP leave uncertainty about the cone photopigments and their distribution, what about approaches using retinal reflection densitometry? Retinal reflection densitometry has the great advantage of being conducted on the living eye under normal physiological conditions. Light is sent through ophthalmic optics and is incident on the cones in the retina from their wider entrance end. Any reflections that return the signal back from the cones to the experimenter are due to coupling to the backwards modes of the cone waveguide structure. The test light probes the cones along a linear axis from the wide end in. Most of the reflection will doubtless occur from this wider, proximal portion of the cones. None-the-less, to some extent the entire length of the cone photosensitive portion can be sampled. But again, is there only one pigment in the cone and if multiple pigments are present, how are they distributed?
Early attempts to characterize cone photopigments by this technique (Ripps and Weale, 1964; Rushton, 1964) suffered from serious signal-to-noise limitations and necessarily had to probe rather large areas of the retina involving many photoreceptors at a time. The technique did provide data on photopigment kinetics (pigment regeneration rates) but were ill-suited to probe individual cones.
The limitation of probe-beam size on the retina was dramatically circumvented in recent years by the techniques developed by the Center for Visual Science group at the University of Rochester. They employed wavefront correction in an adaptive optics technique to illuminate a spot smaller than a single cone in the living eye (Roorda & Williams, 1999). While this approach substantially improves the signal-to-noise problem and also assures the experimenter of probing but a single cone, it still suffers the same limitations mentioned above for any axial probe of the retinal cones. That is, such probing of resident photopigments can not be divorced from waveguide coupling effects and are likely to preferentially probe the proximal regions of the cones. What has been found by this technique is a high preponderance of what appears to be red cone types, fewer green and very few blue. This is perhaps, just what might be expected for probing into a tapered cone waveguide from its wide end where there might (or might not) be a differential distribution of pigment.
Not so incidentally, using a version of this technique, the University of Rochester group also probed the actual sensation elicited from single cones as reported by their subjects. Now, in any three-cone model, illuminating any one cone must produce the sensation of red, green, or blue (depending on which cone “type” was stimulated) regardless of the color of the illuminating light. Instead, they (Hofer, Singer, and Williams, 2005) found that the stimulation of any one cone could elicit any color sensation -- red, yellow, green, blue or even white! These kind of observations make no sense whatsoever in terms of the standard three-cone model of human color vision.
Ok, so even if the ‘evidence’ for three cone classes is equivocal and operation on that basis is directly contradicted by dynamic measurements such as the Ives' result, the model must be a good one since it explains how color vision works anyway, right?
Well, not exactly.